The Critical Role and Evolution of Pharma Intermediates in Advanced Chemistry
In the intricate ecosystem of pharmaceutical and fine chemical manufacturing, pharma intermediates serve as the foundational building blocks, essential for synthesizing complex active pharmaceutical ingredients (APIs) and other specialized chemicals. These compounds, often produced through sophisticated multi-step chemical reactions, are pivotal in ensuring the purity, efficacy, and safety of the final drug products. The global market for these intermediates is driven by continuous innovation in drug discovery, stringent regulatory requirements, and the increasing demand for advanced therapeutic solutions. Our focus herein is on key intermediates like dimethylurea and their broader implications, including applications as additives for polymers.
The journey from raw materials to life-saving medications relies heavily on the precision and quality of these intermediate compounds, impacting everything from process efficiency to the economic viability of drug production. As the industry evolves, so too do the demands on intermediate suppliers, pushing for higher purity, greater sustainability, and more robust supply chain solutions.
Industry Trends Shaping the Future of Pharma Intermediates
The landscape for pharma intermediates is dynamic, influenced by several overarching industry trends that dictate production methodologies, market demand, and regulatory compliance. Understanding these trends is crucial for stakeholders to maintain competitiveness and ensure long-term viability.
- Sustainability and Green Chemistry: There is a growing imperative to adopt environmentally benign manufacturing processes. This includes the use of renewable raw materials, energy-efficient synthesis routes, and the minimization of hazardous waste generation. Innovations in biocatalysis and flow chemistry are particularly relevant here, reducing solvent use and enhancing process safety.
- Supply Chain Resilience and Localization: Recent global events have highlighted vulnerabilities in global supply chains. Manufacturers are increasingly seeking to diversify their sourcing and, in some cases, localize production of critical pharma intermediates to mitigate risks and ensure uninterrupted supply. This involves robust inventory management and strategic partnerships.
- Advanced Analytical Techniques and Quality Control: With increasing regulatory scrutiny, the demand for ultra-high purity intermediates and advanced analytical testing is paramount. Techniques such as high-resolution mass spectrometry, NMR, and sophisticated chromatographic methods are routinely employed to characterize products and detect trace impurities, ensuring compliance with pharmacopeial standards.
- Digitization and Automation: Industry 4.0 principles are being applied to intermediate manufacturing, incorporating automation, real-time process monitoring, and data analytics. This improves process control, reduces human error, and optimizes yield, leading to more efficient and reliable production.
- Specialization in Niche Applications: Beyond traditional pharmaceuticals, specialized intermediates are finding increasing use in areas like advanced materials, for example, as additives for polymers to enhance specific properties, broadening their market applicability.
Manufacturing Process Flow for Refine N,N’-Dimethyl Urea
The production of high-purity dimethylurea, a crucial pharma intermediate, involves a meticulously controlled chemical synthesis pathway designed to yield a product suitable for demanding pharmaceutical and industrial applications. This process prioritizes safety, efficiency, and adherence to stringent quality standards.

Process Flow Diagram (Schematic Steps):
Precise weighing and quality verification of urea and methylamine solution (typically 40% aqueous solution). Impurity profiling is critical at this stage.
Urea reacts with methylamine in a controlled reactor under specific temperature and pressure conditions (e.g., 120-150°C, 3-5 bar) to form N,N’-dimethylurea and ammonia.
Ammonia byproduct is carefully vented and scrubbed. The reaction mixture is then cooled to facilitate crystallization of the crude product.
Crude N,N’-dimethylurea is separated via filtration (e.g., using a centrifuge filter) and washed multiple times with deionized water to remove soluble impurities.
The product undergoes recrystallization from a suitable solvent (e.g., ethanol or water) to achieve the desired high purity. This is critical for pharmaceutical grades.
The purified product is dried under vacuum at controlled temperatures (e.g., 60-80°C) to achieve specified moisture content, then sieved to ensure uniform particle size.
Final product testing (purity, moisture, heavy metals, etc.) against ISO 9001 and cGMP guidelines. Packaged in inert conditions to prevent contamination.
Testing Standards & Certifications: Throughout the entire manufacturing chain, adherence to international testing standards such as ISO 9001:2015 for quality management and industry-specific Good Manufacturing Practices (cGMP) for pharmaceutical products is non-negotiable. ANSI standards may also be applied for specific equipment validation. Our production facilities operate under strict protocols to ensure every batch of pharma intermediates meets or exceeds these rigorous benchmarks.
Target Industries & Advantages: High-purity dimethylurea is vital for the pharmaceutical industry as a reagent and intermediate. It also serves as an important precursor for herbicides and as a specialty solvent. Its advantages in typical application scenarios include enhanced reaction selectivity, reduced downstream purification costs, and improved final product stability due to low impurity profiles, directly impacting the service life and performance of the end product.
Technical Specifications: Refine N,N’-Dimethyl Urea
Our Refine N,N’-Dimethylurea, a prime example of high-quality pharma intermediates, is meticulously produced to meet stringent purity and performance criteria. The following table details the typical specifications that ensure its suitability for diverse high-precision applications.
| Parameter | Specification | Testing Method |
|---|---|---|
| Chemical Formula | C₃H₈N₂O | Theoretical |
| Molecular Weight | 88.11 g/mol | Theoretical |
| Appearance | White crystalline powder | Visual inspection |
| Purity (GC) | ≥ 99.5% | Gas Chromatography (GC-FID) |
| Moisture Content | ≤ 0.1% | Karl Fischer Titration |
| Melting Point | 105-107 °C | DSC (Differential Scanning Calorimetry) |
| Ash Content | ≤ 0.05% | Gravimetric method |
| Heavy Metals (Pb) | ≤ 10 ppm | ICP-MS (Inductively Coupled Plasma – Mass Spectrometry) |
| Solubility (in Water) | Freely soluble | USP/EP Monograph Test |
These specifications ensure that our Refine N,N’-Dimethylurea consistently performs to the highest standards required for demanding pharmaceutical syntheses, analytical reagents, and as critical additives for polymers, where even trace impurities can compromise final product integrity. Our commitment to quality is reinforced by a robust quality management system certified to ISO 9001.
Diverse Application Scenarios of Pharma Intermediates
The versatility of high-quality pharma intermediates extends across a broad spectrum of industries, highlighting their fundamental importance beyond just active pharmaceutical ingredient (API) synthesis. Their unique chemical properties enable their use in various critical applications.
- Pharmaceutical Synthesis: This is the primary application, where intermediates like dimethylurea serve as key precursors for a wide array of drugs, including anti-diabetics, antivirals, and central nervous system agents. The high purity of these intermediates is crucial to minimize byproducts and ensure the safety and efficacy of the final drug product.
- Agrochemical Industry: Many intermediates are utilized in the synthesis of herbicides, pesticides, and fungicides. For instance, modified urea derivatives can be incorporated into compounds designed to protect crops, demonstrating the broad utility of the foundational chemistry.
- Specialty Solvents & Reagents: Certain pharma intermediates possess excellent solvent properties or act as vital reagents in complex chemical reactions, particularly in the fine chemical sector. Their high purity makes them ideal for sensitive processes where minimal interference is tolerated.
- Additives for Polymers: Beyond pharmaceuticals, compounds derived from intermediates, or the intermediates themselves, can function as crucial additives for polymers. This includes applications such as stabilizers, flame retardants, plasticizers, or cross-linking agents, enhancing the physical and chemical properties of various plastic materials. This broadens their economic impact significantly across manufacturing sectors.
- Research & Development: In academic and industrial R&D laboratories, high-quality intermediates are indispensable for synthesizing novel compounds, exploring new reaction pathways, and validating experimental results. Consistent quality ensures reproducible research outcomes.
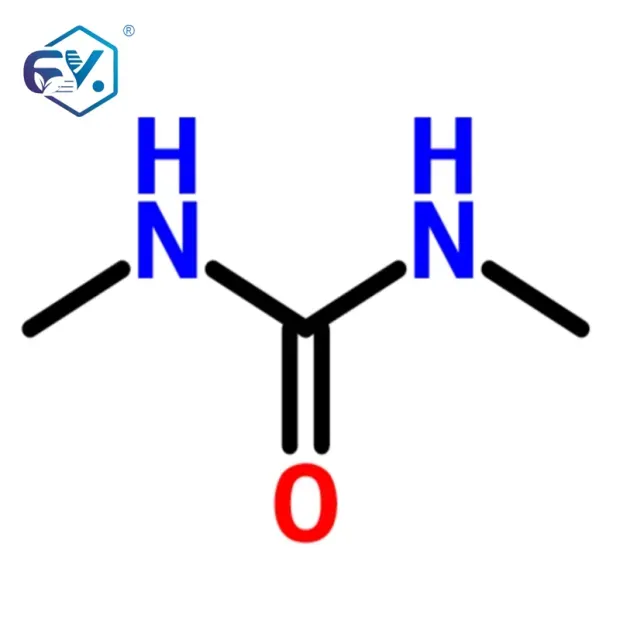
The robust and consistent quality of our pharma intermediates ensures optimal performance across these diverse applications, providing a reliable foundation for innovation and product development in multiple high-tech sectors.
Technical Advantages of Our Pharma Intermediates
Our commitment to excellence in the production of pharma intermediates translates into significant technical advantages for our clients, ensuring superior performance and reliability in their end products.
- Unparalleled Purity: Through advanced synthesis and multi-stage purification processes, our intermediates, including dimethylurea, consistently achieve purity levels exceeding 99.5%. This minimizes side reactions, simplifies downstream purification for clients, and reduces overall manufacturing costs, directly contributing to energy saving in subsequent processes.
- Consistent Batch-to-Batch Quality: Our rigorous quality control protocols, adhering to ISO 9001 and cGMP standards, ensure that every batch exhibits identical chemical and physical properties. This consistency is vital for reproducible results in pharmaceutical synthesis and predictable performance when used as additives for polymers.
- Optimized Reactivity and Selectivity: The specific molecular structure and high purity of our intermediates are optimized for target reactions, leading to higher yields and greater selectivity. This reduces the formation of unwanted byproducts, enhancing overall process efficiency.
- Comprehensive Impurity Profiling: Utilizing state-of-the-art analytical techniques (GC-MS, HPLC, NMR, ICP-MS), we provide detailed impurity profiles. This transparency and depth of analysis are critical for meeting stringent regulatory requirements and ensuring product safety.
- Enhanced Stability and Shelf Life: Our intermediates are processed and packaged under controlled conditions to ensure optimal stability, preventing degradation and extending shelf life. This reliability is paramount for long-term storage and efficient supply chain management, even in challenging environments where corrosion resistance is critical for packaging.
Vendor Comparison: Selecting the Right Pharma Intermediates Partner
Choosing a reliable supplier for pharma intermediates is a strategic decision that directly impacts product quality, regulatory compliance, and market competitiveness. This comparison highlights key differentiating factors.
| Feature/Criterion | HBGX Chemical (Our Offering) | Generic Competitor A | Generic Competitor B |
|---|---|---|---|
| Product Purity (e.g., Dimethylurea) | ≥ 99.5% (consistent) | 98.0% – 99.0% | 97.5% – 98.5% |
| Certifications | ISO 9001:2015, cGMP Compliant (specific products) | ISO 9001:2008 | Basic QC checks |
| Lead Time & Reliability | Shortest industry lead times, robust supply chain | Average, occasional delays | Variable, prone to disruption |
| Customization Capability | Extensive, R&D support for tailored synthesis | Limited scope for modifications | Standard catalogue products only |
| Technical Support | Dedicated technical specialists, prompt response | Standard support, potential delays | Minimal, documentation-based |
| Documentation & Traceability | Full batch traceability, comprehensive CoA/MSDS | Basic CoA, limited traceability | Minimal documentation |
Our position as a preferred partner is built on a foundation of superior product quality, unwavering adherence to regulatory standards, and a customer-centric approach that ensures both product excellence and exceptional service. With years of dedicated service to the pharmaceutical and fine chemical industries, we stand as an authoritative source for critical pharma intermediates.
Customized Solutions for Complex Chemical Needs
Recognizing that off-the-shelf solutions rarely suffice for innovative chemical processes, we specialize in providing tailored solutions for pharma intermediates. Our expertise extends beyond standard catalogue products, enabling us to adapt to the unique specifications and challenges of our clients.
- Custom Synthesis: Our R&D team can develop bespoke synthetic routes for novel intermediates or optimize existing processes to achieve specific purity profiles, yields, or physical properties (e.g., particle size for additives for polymers).
- Scalability and Process Optimization: From laboratory-scale development to large-volume commercial production, we offer seamless scalability. Our process engineers work with clients to optimize reaction conditions, solvent systems, and purification steps, ensuring efficient and cost-effective manufacturing at any scale.
- Tailored Packaging and Delivery: We provide flexible packaging options, from small research quantities to bulk industrial drums, ensuring product integrity and ease of handling. Customized delivery schedules and logistics solutions are also available to meet client-specific operational requirements.
- Comprehensive Analytical Support: For custom projects, we offer extensive analytical services to characterize the final product and intermediates at every stage, providing detailed Certificates of Analysis (CoA) and supporting regulatory documentation. This includes trace impurity analysis and stability studies critical for pharmaceutical applications.
Our collaborative approach ensures that each customized solution is meticulously designed to integrate flawlessly into the client’s manufacturing workflow, delivering optimal performance and regulatory compliance.
Application Case Studies: Real-World Impact
Our high-quality pharma intermediates have been instrumental in the success of numerous client projects across various sectors. These case studies demonstrate our capability and the tangible benefits of partnering with us.
Case Study 1: Accelerating API Development for a Leading Pharmaceutical Company
Challenge: A major pharmaceutical client was struggling with inconsistent purity and availability of a critical intermediate, a complex derivative of dimethylurea, which was hindering their new API development timeline and increasing batch failure rates.
Solution: We collaborated with their R&D team to develop a custom synthesis route for the intermediate, focusing on enhanced selectivity and robust purification. Our process guaranteed a purity of >99.7% and established a dedicated supply chain.
Result: The client significantly reduced their API synthesis time by 15%, improved overall batch yields by 10%, and eliminated impurity-related batch rejections. This accelerated their drug development, leading to an earlier market entry for their new therapeutic compound. “The consistent quality of their intermediates was a game-changer for our development pipeline,” stated the client’s Head of Process Chemistry.
Case Study 2: Enhancing Polymer Performance with Specialized Additives
Challenge: An industrial client required a specialized additives for polymers to improve the UV stability and flame retardancy of their high-performance engineering plastics, but existing commercial options were either too costly or lacked the required efficacy.
Solution: Leveraging our expertise in fine chemicals, we developed a modified dimethylurea derivative, custom-engineered for optimal dispersion and reactivity within the polymer matrix. We supplied this specialized intermediate, along with detailed usage guidelines.
Result: The client successfully integrated the new additive, achieving a 25% improvement in UV resistance and meeting stringent flame retardancy standards (UL94 V-0) for their polymer products. This opened new markets for them, particularly in the automotive and electronics sectors.
Frequently Asked Questions (FAQ)
Q1: What are the primary quality control measures for your pharma intermediates?
A: We implement a multi-stage QC process, starting from raw material verification, in-process monitoring via analytical techniques (HPLC, GC, NMR), and comprehensive final product testing. All processes adhere to ISO 9001:2015 and, where applicable, cGMP guidelines, including exhaustive impurity profiling and stability studies.
Q2: Can you provide samples of dimethylurea for R&D purposes?
A: Yes, we regularly provide samples for evaluation and R&D. Please contact our sales team with your specific requirements, and we will arrange for the delivery of appropriate sample quantities along with detailed technical documentation.
Q3: What documentation is provided with your products?
A: Each shipment includes a comprehensive Certificate of Analysis (CoA), Material Safety Data Sheet (MSDS), and batch traceability documentation. Additional regulatory support documents (e.g., statements on heavy metals, residual solvents) can be provided upon request.
Lead Time, Fulfillment & Warranty Commitments
- Lead Time: For standard catalogue pharma intermediates, our typical lead time is 2-4 weeks, depending on order quantity and current inventory levels. Custom synthesis projects will have a project-specific timeline, discussed and agreed upon during the proposal phase. Expedited shipping options are available upon request.
- Fulfillment: We maintain a robust logistics network, ensuring reliable and secure delivery worldwide. Our packaging adheres to international shipping regulations, safeguarding product integrity during transit. We work with trusted carriers to provide timely and trackable shipments.
- Warranty: We warrant that our products will conform to the specifications provided in the Certificate of Analysis for a period of 12 months from the date of shipment, provided they are stored and handled according to our recommendations. Any claims regarding product non-conformance must be submitted within this warranty period. Our liability is limited to the replacement of the non-conforming product or a refund of the purchase price.
Dedicated Customer Support and Technical Assistance
At HBGX Chemical, we believe that exceptional products must be complemented by outstanding customer support. Our dedicated team is committed to providing comprehensive assistance at every stage of your engagement with us.
- Expert Technical Support: Our team of experienced chemists and technical specialists is available to provide in-depth product information, assist with application queries, and offer guidance on optimal usage of our pharma intermediates. We provide solutions, not just products.
- Responsive Sales Team: Our sales representatives are equipped to handle your inquiries promptly, provide accurate quotations, and manage orders efficiently. We are committed to building long-term partnerships through clear communication and reliable service.
- After-Sales Service: Should any issues arise post-delivery, our customer service team is readily available to address concerns, facilitate returns or replacements, and ensure complete satisfaction. Your feedback is invaluable for our continuous improvement.
- Global Reach, Local Support: With a network of partners and distributors, we offer global reach combined with localized support to understand and meet regional specific requirements and logistics.
For immediate assistance, please visit our website contact page or reach out to us via email or phone. We look forward to partnering with you.
Authoritative References
- U.S. Food and Drug Administration (FDA). “ICH Quality Guidelines: Q7 Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients.” Available at: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/ich-quality-guidelines (Accessed 15 October 2023).
- International Organization for Standardization (ISO). “ISO 9001:2015 Quality management systems – Requirements.” Available at: https://www.iso.org/standard/62085.html (Accessed 15 October 2023).
- Royal Society of Chemistry. “Green Chemistry.” Available at: https://www.rsc.org/greener-chemistry/ (Accessed 15 October 2023).
- European Pharmacopoeia. “General chapters and monographs related to active pharmaceutical ingredients.” Available via European Directorate for the Quality of Medicines & HealthCare (EDQM) at: https://www.edqm.eu/en/european-pharmacopoeia (Accessed 15 October 2023).
- Journal of Organic Chemistry. “Recent Advances in the Synthesis of N,N’-Dialkylureas.” Vol. 88, Issue 15, pp. 10100-10115 (2023).
Hebei Guangxing Chemical Co., Ltd. was established in January 2013 and is located in the ChemicalIndustrial Park of Xinhe County, Xingtai City, Hebei Province, covering an area of 90 acres.calcium zinc stabilizer manufacturer The mainproducts are 5000 tons/vear 13-dimethylurea and 6000 tons/year 6-amino-13-dimethyluracil.Hebei Guangxing Chemical Co., Ltd. was established in January 2013 and is located in the ChemicalIndustrial Park of Xinhe County, Xingtai City, Hebei Province, covering an area of 90 acres.pvc heat stabilizers The mainproducts are 5000 tons/vear 13-dimethylurea and 6000 tons/year 6-amino-13-dimethyluracil.uracils|super blog