The Crucial Role of Pharmaceutical Intermediates in Modern Drug Development
In the dynamic landscape of pharmaceutical manufacturing, the role of pharma intermediates is paramount. These compounds, essential building blocks in the synthesis of active pharmaceutical ingredients (APIs), dictate the quality, efficacy, and safety of final drug products. The global pharmaceutical industry is experiencing unprecedented growth, driven by an aging population, increasing prevalence of chronic diseases, and continuous innovation in drug discovery. This escalating demand places immense pressure on the supply chain for high-quality chemical precursors, emphasizing the need for reliable, compliant, and technically advanced suppliers.
Key industry trends underscore this evolution. Firstly, there’s a pronounced shift towards specialized and complex molecules, requiring bespoke synthesis routes and highly purified intermediates. Secondly, regulatory scrutiny, particularly from bodies like the FDA and EMA, is intensifying, demanding rigorous adherence to cGMP (current Good Manufacturing Practices) and comprehensive documentation throughout the supply chain. Thirdly, sustainability and green chemistry principles are gaining traction, pushing manufacturers to adopt environmentally friendly processes and materials. Compounds like N,N’-Dimethyl Urea, often utilized as a crucial reagent or intermediate, exemplify the precision required in this sector, serving as precursors for a range of pharmaceutical compounds and sometimes even as an intermediate in specialized polymer applications as additives for polymers. Our focus here is to provide B2B decision-makers and engineers with a comprehensive understanding of the value proposition offered by meticulously produced pharma intermediates.
The Precision Manufacturing Process of Pharma Intermediates
The production of essential pharmaceutical building blocks is a complex, multi-stage process demanding absolute precision, stringent quality control, and robust adherence to pharmaceutical standards. Our manufacturing methodology ensures the highest purity and consistency, critical for downstream API synthesis.
Process Flow Overview:
- Raw Material Sourcing & Pre-treatment: High-grade raw materials are meticulously selected from qualified suppliers. For products like Refine N,N’-Dimethyl Urea, precursor chemicals such as methylamine and carbon dioxide are sourced with documented purity and traceability. Pre-treatment may involve drying, filtration, or specific purifications to remove trace impurities that could impact the final product.
- Reaction/Synthesis: This is the core stage where chemical transformations occur. For N,N’-Dimethyl Urea, this typically involves the reaction of methylamine with a suitable carbonyl source under controlled temperature and pressure conditions. Advanced catalytic synthesis techniques are employed to maximize yield, selectivity, and minimize undesirable by-products. Parameters like temperature, pressure, stirring speed, and reactant feed rates are precisely monitored and controlled using sophisticated PLC systems, preventing side reactions and ensuring product integrity.
- Purification & Isolation: Following synthesis, the crude product undergoes rigorous purification. This often involves a combination of techniques such as crystallization, distillation, solvent extraction, and chromatography. For example, fractional distillation might be used to separate N,N’-Dimethyl Urea from solvents and unreacted raw materials, while subsequent crystallization steps achieve the desired purity profile and crystal morphology. Multi-stage filtration processes remove particulate matter, ensuring a clean product.
- Drying & Milling: The purified product is then dried under controlled conditions to achieve specific moisture content, preventing degradation and ensuring stability. Techniques like vacuum drying or fluid bed drying are common. If required, the product may be milled to a specific particle size distribution to meet formulation requirements, particularly for solid-dose applications.
- Quality Control & Analysis: Throughout the entire process, and especially at the final stage, comprehensive quality control (QC) is performed. This includes testing for assay, impurity profile, residual solvents, heavy metals, moisture content, and physical characteristics.
- Packaging & Storage: The final product is packaged in cGMP-compliant materials under inert atmosphere, if necessary, to maintain stability. Secure and temperature-controlled storage ensures product integrity until shipment.

Figure 1: Schematic representation of a typical manufacturing facility for pharmaceutical intermediates.
Key Technical Specifications & Standards:
- Testing Standards: All products adhere to international pharmacopoeia standards such as USP (United States Pharmacopeia), EP (European Pharmacopoeia), and JP (Japanese Pharmacopoeia), as applicable. Manufacturing facilities are ISO 9001:2015 certified for quality management systems and operate under strict cGMP guidelines. Environmental management adheres to ISO 14001, and occupational health and safety to ISO 45001.
- Materials of Construction: Reaction vessels, piping, and storage tanks are typically constructed from pharmaceutical-grade stainless steel (e.g., SS316L) to ensure inertness, prevent contamination, and offer superior corrosion resistance, especially vital for handling corrosive reagents or products.
- Service Life & Durability: Our equipment and processes are designed for long-term reliability and robustness. For example, specialized linings and coatings are applied in areas susceptible to chemical attack, extending the service life of critical components and ensuring consistent production quality over years of operation.
Target Industries & Advantages:
Our high-purity chemical intermediates serve a broad spectrum of industries beyond traditional pharmaceuticals, including specialized chemical synthesis and advanced materials.
- Pharmaceutical & Biopharmaceutical: Primary sector, providing precursors for APIs in therapeutic areas such as oncology, cardiovascular diseases, CNS disorders, and anti-infectives.
- Specialty Chemicals: Supplying high-purity reagents and building blocks for fine chemical synthesis, including advanced agrochemicals.
- Polymers & Materials Science: In specific cases, some intermediates, like high-purity dimethylurea, can act as crucial additives for polymers, enhancing properties like flame retardancy or stability, or as key monomers for specialty polymers.
Advantages in these scenarios include enhanced product performance, energy saving during client’s downstream processing due to optimized intermediate properties, and superior corrosion resistance of the final products derived from our intermediates, leading to extended shelf-life and stability.
Technical Specifications: Refine N,N’-Dimethyl Urea (DMU)
Refine N,N’-Dimethyl Urea (DMU) is a prime example of a versatile pharma intermediates, crucial in various organic synthesis routes. Its high purity and consistent quality make it indispensable for demanding pharmaceutical and specialty chemical applications.
Product Specifications: Refine N,N’-Dimethyl Urea
| Parameter | Specification | Method |
|---|---|---|
| Chemical Name | N,N’-Dimethyl Urea | Internal Standard |
| CAS Number | 96-31-1 | |
| Molecular Formula | C3H8N2O | |
| Molecular Weight | 88.11 g/mol | |
| Appearance | White Crystalline Powder | Visual |
| Purity (Assay) | ≥ 99.5% (HPLC) | HPLC |
| Melting Point | 105-108 °C | USP <741> |
| Moisture Content | ≤ 0.5% | Karl Fischer |
| Residue on Ignition | ≤ 0.1% | USP <281> |
| Heavy Metals (Pb) | ≤ 10 ppm | ICP-MS |
| Solubility | Soluble in water, ethanol, chloroform |
These stringent specifications ensure that our Refine N,N’-Dimethyl Urea meets the exacting requirements for pharmaceutical synthesis, contributing to the development of safe and effective medications. The low impurity profile and high assay are critical for minimizing purification steps in API manufacturing and preventing potential side reactions.
Diverse Application Scenarios for High-Quality Pharma Intermediates
The versatility of high-quality pharmaceutical building blocks like Refine N,N’-Dimethyl Urea extends across a wide array of pharmaceutical and industrial applications. Their reliable chemical properties and purity are indispensable for various synthesis pathways.
Primary Pharmaceutical Applications:
- API Synthesis: As fundamental building blocks for Active Pharmaceutical Ingredients, these intermediates are integral to creating new drug molecules. For instance, dimethylurea is a key starting material or reagent in the synthesis of various pharmaceutical compounds, including certain sedatives, antiepileptics, and specific cardiovascular agents. Its reactive groups enable facile derivatization to complex API structures.
- Drug Formulation: While primarily used in synthesis, the purity of intermediates indirectly impacts the final formulation’s stability and efficacy. Residual impurities from low-grade intermediates can lead to degradation products in the final drug, affecting its shelf-life and patient safety.
- Custom Synthesis & R&D: Pharmaceutical R&D heavily relies on a diverse range of intermediates for synthesizing novel compounds for clinical trials. High-purity, well-characterized intermediates accelerate drug discovery by providing reliable starting materials for complex reaction schemes.
- Veterinary Medicine: Similar to human pharmaceuticals, veterinary drug development also requires high-grade intermediates to ensure the safety and efficacy of medications for animals, from antibiotics to parasiticides.
Beyond Pharmaceuticals: Industrial Applications
- Specialty Chemical Manufacturing: High-purity intermediates find use in the production of other fine chemicals, including agrochemicals, dyes, and performance materials, where precise molecular structures are critical.
- Polymer Additives: Certain chemical intermediates, including specific derivatives of dimethylurea, exhibit properties that make them valuable additives for polymers. These can function as flame retardants, plasticizers, or stabilizers, enhancing the physical and chemical properties of a wide range of polymeric materials used in industries from automotive to construction. The high purity translates directly to better performance and predictability in the polymer matrix.
- Research & Laboratory Use: Academic and industrial research laboratories utilize these intermediates as reagents and standards for various chemical experiments and analytical procedures, demanding extreme purity for accurate results.
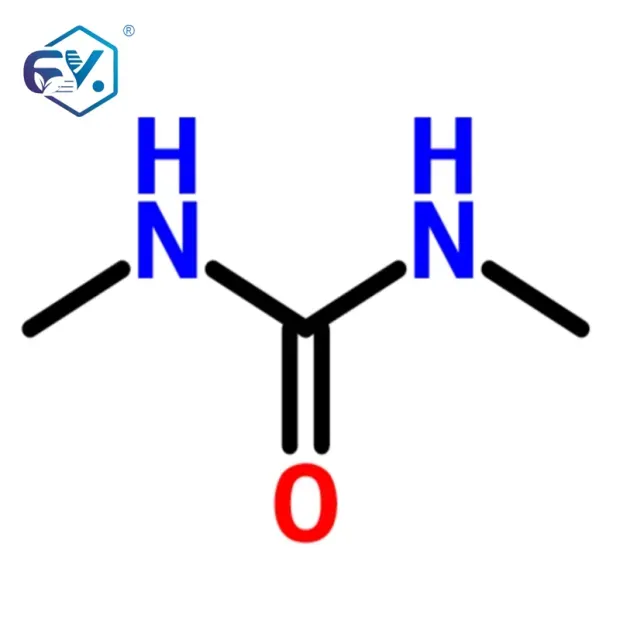
Figure 2: Diverse application landscape for high-quality chemical intermediates across pharmaceutical and industrial sectors.
The breadth of these applications highlights why a consistent supply of meticulously manufactured pharmaceutical precursors is not just beneficial, but absolutely vital for innovation and production across multiple high-tech industries.
Technical Advantages and Value Proposition
Investing in high-quality pharma intermediates offers substantial technical and economic advantages for pharmaceutical manufacturers and specialty chemical producers. Our commitment to excellence translates into tangible benefits for our partners.
Core Advantages:
- Unrivaled Purity and Consistency: Our multi-stage purification protocols, including advanced crystallization and chromatography, ensure impurity profiles consistently below detection limits for critical contaminants. This high purity directly reduces downstream purification efforts and costs for API manufacturers, minimizing batch failures and re-work. Each batch of dimethylurea, for instance, exhibits identical physical and chemical properties, guaranteeing predictable reaction kinetics and yields.
- Optimized Reaction Performance: Consistent quality translates to optimized reaction performance in synthesis. Reduced variability in intermediate purity means less catalyst poisoning, fewer side reactions, and higher overall synthetic yields, contributing to significant energy saving during the client’s production processes.
- Enhanced Regulatory Compliance: Our adherence to cGMP, ISO, and pharmacopoeia standards ensures a robust regulatory dossier for our intermediates. This proactive compliance significantly streamlines the regulatory approval process for our clients’ final drug products, reducing time-to-market and associated costs.
- Superior Stability and Shelf-Life: Strict control over moisture, residual solvents, and oxidative impurities results in intermediates with excellent long-term stability. This prolongs shelf-life, reduces waste, and provides greater flexibility in production scheduling for our clients.
- Reduced Environmental Impact: Employing advanced synthesis technologies and efficient purification methods not only improves product quality but also leads to reduced solvent usage, lower energy consumption, and minimized waste generation in our own operations, aligning with global sustainability objectives.
- Technical Support and Expertise: Our team of experienced chemists and engineers provides unparalleled technical support, assisting clients with process optimization, troubleshooting, and regulatory guidance, fostering a collaborative partnership.
By choosing our premium pharmaceutical precursors, partners gain not just a chemical compound, but a comprehensive solution that reduces risks, optimizes processes, and ultimately contributes to the successful and timely delivery of critical drug products to market. The technical superiority of our products, such as Refine N,N’-Dimethyl Urea, ensures maximum value at every stage of the pharmaceutical value chain.
Vendor Comparison: Choosing the Right Partner for Pharmaceutical Precursors
Selecting a supplier for critical pharmaceutical chemical building blocks is a strategic decision that impacts the entire drug development and manufacturing lifecycle. It goes beyond mere price points; it encompasses quality, reliability, and technical partnership.
Key Criteria for Vendor Evaluation:
- Quality Certifications & Compliance: Does the vendor hold cGMP, ISO 9001, ISO 14001, and ISO 45001 certifications? Are their products compliant with relevant pharmacopoeia standards (USP, EP, JP)?
- Technical Capabilities & R&D: What are their R&D investments? Can they offer custom synthesis? Do they have a deep understanding of complex chemical synthesis routes?
- Production Capacity & Scalability: Can they reliably supply quantities from kilograms to metric tons? Do they have redundant production lines or multiple facilities to ensure supply security?
- Supply Chain Reliability: How robust is their raw material sourcing? What are their lead times and logistics capabilities?
- Quality Control & Analytics: What analytical techniques do they employ (HPLC, GC-MS, NMR, ICP-MS)? How comprehensive is their COA (Certificate of Analysis)?
- Customer Support & Responsiveness: How quickly do they respond to inquiries? Do they offer dedicated technical support?
Comparative Analysis: Premium vs. Standard Suppliers for Refine N,N’-Dimethyl Urea
| Feature/Parameter | Premium Supplier (e.g., HBGX Chemical) | Standard Supplier |
|---|---|---|
| Purity (N,N’-Dimethyl Urea) | ≥ 99.5% (HPLC, consistent batch-to-batch) | ≥ 98.0% (variable purity, higher impurities) |
| Impurity Profile | Extremely low and well-characterized; no problematic contaminants | Higher levels, less characterized; potential for unknown impurities |
| Regulatory Documentation | Comprehensive cGMP, ICH Q7, DMF support, full audit access | Basic COA, limited regulatory support |
| Supply Chain Security | Multiple qualified raw material sources, robust inventory management, global logistics | Single source dependence, limited inventory, local logistics only |
| Technical Support | Dedicated team of chemists, process optimization assistance, troubleshooting | Limited to sales support, no scientific guidance |
| Price Point (relative) | Higher initial cost, but lower total cost of ownership (TCO) due to reduced risks and processing. | Lower initial cost, but higher TCO due to quality issues, re-work, and regulatory hurdles. |
The choice of a premium supplier, despite a potentially higher upfront cost, ultimately leads to significant savings and reduced risks across the entire pharmaceutical value chain. Our commitment to excellence, coupled with robust quality systems and unparalleled technical support, positions us as the preferred partner for critical pharmaceutical manufacturing components.
Customized Solutions and Collaborative Partnership
Recognizing that every pharmaceutical project has unique requirements, we offer highly customized solutions for chemical intermediates. Our approach centers on deep collaboration to meet specific technical, regulatory, and logistical needs.
Tailored Services Include:
- Custom Synthesis & Process Development: For novel APIs or proprietary synthesis routes, our R&D team works closely with clients to develop and optimize the manufacturing process for specific intermediates. This includes route scouting, process scale-up from gram to multi-ton quantities, and impurity profiling to meet unique specifications.
- Impurity Identification & Control: We offer advanced analytical services for identifying and quantifying trace impurities, crucial for regulatory submissions. We can develop methods to control or remove specific impurities as per client requirements, ensuring compliance with strict limits.
- Flexible Packaging & Logistics: Customized packaging solutions, including specific container111 types, sizes, and inert atmosphere packing, are available to suit client’s handling and storage protocols. We also offer tailored logistics, including just-in-time delivery and cold chain options, to ensure seamless integration into manufacturing schedules.
- Regulatory Support: Our dedicated regulatory affairs team assists with documentation required for filings, including Letters of Access (LoA) for Drug Master Files (DMFs), certificates of origin, and compliance statements, accelerating market authorization.
- Quality by Design (QbD) Integration: We can integrate QbD principles into the development and manufacturing of intermediates, proactively designing quality into the product and process, leading to more robust and consistent outcomes.
Our commitment to customized solutions solidifies our role as a strategic partner, not just a supplier. By addressing specific challenges and aligning with client objectives, we help accelerate drug development cycles and enhance overall product quality.
Application Case Studies: Proving Our Expertise
Our expertise in providing high-quality pharmaceutical precursors is demonstrated through successful collaborations with leading pharmaceutical companies worldwide. These case studies highlight our ability to deliver critical materials that accelerate drug development and ensure product integrity.
Case Study 1: Accelerating Oncology Drug Development
A global pharmaceutical company was developing a novel oncology API that required a highly specialized and enantiomerically pure intermediate. Their previous supplier struggled with batch-to-batch consistency and high levels of a critical impurity, leading to significant delays in clinical trials. We engaged with their R&D team, providing a high-purity chemical intermediate synthesized using an optimized, proprietary route. Our product consistently exceeded their purity requirements (≥ 99.8% enantiomeric excess and <0.05% critical impurity), demonstrating exceptional batch uniformity. This not only enabled the client to resume their clinical trials promptly but also reduced their purification steps for the API by 30%, resulting in substantial cost savings and an expedited path to regulatory submission. This successful partnership underscored our capability in complex synthesis and stringent quality control.
Case Study 2: Enhancing Veterinary Antibiotic Efficacy with High-Purity Dimethylurea
A major veterinary pharmaceutical manufacturer faced challenges with the stability and bioavailability of a broad-spectrum antibiotic. Investigation traced the issue back to a trace impurity in their dimethylurea supply, which was acting as a catalyst for degradation over time. Upon switching to our Refine N,N’-Dimethyl Urea, characterized by its exceptionally low impurity profile and controlled moisture content, the client observed a remarkable improvement. The degradation rate of their antibiotic formulation decreased by 50% over a 24-month stability study. This enhanced stability translated into an extended shelf-life for their final product, reduced recall risks, and ultimately, improved efficacy and trust among veterinary practitioners. This highlights how the superior quality of a fundamental intermediate like dimethylurea can have a profound impact on downstream product performance and market success.
Trustworthiness and Support: Our Commitment to Clients
At the core of our operations is a steadfast commitment to client trust, backed by transparent practices and comprehensive support services. We understand that reliability is paramount in the pharmaceutical industry.
Frequently Asked Questions (FAQ):
Q: What quality certifications do you hold for your pharmaceutical intermediates?
A: Our manufacturing facilities are ISO 9001:2015 certified, and all relevant products are produced under cGMP guidelines. We also ensure compliance with specific pharmacopoeia standards (USP, EP, JP) and provide comprehensive documentation including Certificates of Analysis (CoA), MSDS, and support for Drug Master Files (DMFs).
Q: Can you accommodate custom synthesis requests for specific intermediates?
A: Yes, we specialize in custom synthesis and process development. Our R&D team works closely with clients from laboratory scale to commercial production, ensuring tailored solutions for unique molecular structures or specific purity profiles.
Q: What is your typical lead time for orders?
A: Lead times vary depending on product availability, quantity, and specific customization requirements. For standard products like Refine N,N’-Dimethyl Urea, we typically fulfill orders within 2-4 weeks. Custom synthesis projects will have a project-specific timeline communicated upfront.
Q: What kind of after-sales support do you provide?
A: We offer extensive after-sales support, including dedicated technical assistance from our team of chemists, regulatory guidance, and responsive customer service for any logistics or product-related inquiries. We are committed to resolving any issues swiftly and efficiently.
Lead Time & Fulfillment Details:
Our robust supply chain and optimized production planning enable efficient order fulfillment. Standard bulk orders for high-demand pharmaceutical intermediates are typically processed and ready for shipment within 14-28 calendar days. For urgent requirements or specialized products, expedited services can be arranged. We leverage global logistics networks to ensure timely and secure delivery to your facility, with full traceability from our plant to your dock.
Warranty Commitments:
We stand by the quality of our chemical building blocks. All products are warranted to meet or exceed the specifications provided in their respective Certificates of Analysis (CoA) and comply with agreed-upon pharmacopoeial standards at the time of shipment. In the rare event of a product not meeting these specifications, we are committed to prompt investigation, replacement, or credit, in accordance with our quality assurance protocols and mutual agreement. Our quality management system includes rigorous batch retention and testing to support any investigations.
Customer Support:
Our dedicated customer support team and technical experts are available to assist you throughout your journey. From initial inquiry and technical consultation to order placement and post-delivery support, we ensure a seamless and responsive experience. Contact us via our website, email, or direct phone lines for expert assistance. We pride ourselves on building long-term, trust-based relationships with our clients, underpinned by reliable product quality and exceptional service.
Conclusion
The demand for high-purity pharma intermediates continues to grow, reflecting the increasing complexity and regulatory demands of the global pharmaceutical industry. As a dedicated provider, we are committed to delivering superior quality, technical excellence, and unwavering support to our partners. Our Refine N,N’-Dimethyl Urea exemplifies this commitment, offering unmatched purity and consistency vital for critical applications. By partnering with us, pharmaceutical manufacturers can ensure the integrity of their supply chain, accelerate drug development, and confidently bring safe and effective medicines to market.
References:
- United States Pharmacopeial Convention. (2023). United States Pharmacopeia and National Formulary (USP-NF).
- European Directorate for the Quality of Medicines & HealthCare (EDQM). (2023). European Pharmacopoeia.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). (2009). ICH Q7: Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients.
- Food and Drug Administration (FDA). (2019). Guidance for Industry: ICH Q7 Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients.
- ISO. (2015). ISO 9001: Quality management systems – Requirements.
Hebei Guangxing Chemical Co., Ltd. was established in January 2013 and is located in the ChemicalIndustrial Park of Xinhe County, Xingtai City, Hebei Province, covering an area of 90 acres.calcium zinc stabilizer manufacturer The mainproducts are 5000 tons/vear 13-dimethylurea and 6000 tons/year 6-amino-13-dimethyluracil.Hebei Guangxing Chemical Co., Ltd. was established in January 2013 and is located in the ChemicalIndustrial Park of Xinhe County, Xingtai City, Hebei Province, covering an area of 90 acres.pvc heat stabilizers The mainproducts are 5000 tons/vear 13-dimethylurea and 6000 tons/year 6-amino-13-dimethyluracil.uracils|super blog