Industry Trends and Market Dynamics for Chlorhexidine
The global market for Chlorhexidine, a broad-spectrum antimicrobial agent, continues to demonstrate robust growth driven by increasing demand in healthcare, personal care, and veterinary sectors. Its efficacy against a wide range of gram-positive and gram-negative bacteria, fungi, and some viruses makes it indispensable for infection control and antiseptic applications. Recent trends highlight a push towards higher purity grades, enhanced formulation stability, and sustainable manufacturing practices in response to evolving regulatory landscapes and environmental concerns. The market is also seeing innovation in delivery systems, with a focus on extended-release formulations and combination products to enhance therapeutic outcomes and patient compliance. According to a report by Grand View Research, the global Chlorhexidine market size was valued at USD 178.6 million in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 6.5% from 2023 to 2030, underscoring its critical role in public health initiatives worldwide.
Regulatory bodies like the FDA and EMA maintain stringent standards for product quality and safety, requiring manufacturers to adhere to Good Manufacturing Practices (GMP) and conduct rigorous testing. This has led to a consolidation among major suppliers capable of meeting these elevated benchmarks, favoring those with established quality control systems and proven production capabilities. Furthermore, the rising awareness about hospital-acquired infections (HAIs) and the increasing adoption of preventive measures in surgical settings and intensive care units are significant market drivers, reinforcing the demand for reliable and effective antiseptic solutions based on Chlorhexidine.
Detailed Manufacturing Process Flow of Chlorhexidine
The synthesis of Chlorhexidine, specifically Chlorhexidine digluconate or dihydrochloride, is a sophisticated chemical process requiring precise control over reaction parameters and purification steps to ensure a high-purity, pharmaceutical-grade product. The primary raw material for its synthesis is 4-chloroaniline, which undergoes a series of reactions to form the diguanide structure.
Process Flow Schematic Steps:
- Step 1: Guanidination of 4-Chloroaniline
4-Chloroaniline is reacted with dicyandiamide in the presence of an acid catalyst, typically under elevated temperature and pressure, to form 1-(4-chlorophenyl)-3-cyanoguanidine. This step involves the addition of the guanidine functional group. - Step 2: Dimerization
The intermediate from Step 1 is then reacted with another molecule of 4-chloroaniline and a coupling agent to form the bis-guanide structure, specifically N,N’-bis(4-chlorophenyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediimidamide. This is the core molecular backbone of Chlorhexidine. - Step 3: Salt Formation (e.g., Digluconate or Dihydrochloride)
The crude Chlorhexidine base is highly insoluble. To make it water-soluble and suitable for formulation, it is reacted with an appropriate acid. For Chlorhexidine digluconate, gluconic acid is used, forming a stable, water-soluble salt. For Chlorhexidine dihydrochloride, hydrochloric acid is used. - Step 4: Purification and Crystallization
The salt solution undergoes multiple purification steps, including activated carbon treatment, filtration, and recrystallization, to remove impurities, unreacted starting materials, and by-products. This ensures the high purity and pharmaceutical grade required for medicinal applications. - Step 5: Drying and Milling
The purified crystalline product is then dried under controlled conditions to remove residual solvents and moisture. It may then be milled to achieve a specific particle size distribution, depending on the final application. - Step 6: Quality Control & Packaging
Every batch undergoes rigorous quality control testing.
Testing Standards and Quality Assurance:
Our manufacturing processes adhere strictly to international quality management systems, including ISO 9001:2015 for quality management and ISO 14001:2015 for environmental management. All batches of Chlorhexidine are tested against pharmacopeial standards such as the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and British Pharmacopoeia (BP) for purity, assay, impurities profile, and physical characteristics. Typical tests include HPLC for assay and related substances, Karl Fischer for moisture content, and heavy metals analysis. Our products demonstrate exceptional stability, ensuring a service life typically exceeding 3 years under recommended storage conditions, critical for industries where long shelf-life and consistent efficacy are paramount.
Target Industries and Advantages:
High-purity Chlorhexidine is critical for the pharmaceutical, cosmetic, and veterinary industries. Its advantages in typical application scenarios include:
- Healthcare: Superior broad-spectrum antiseptic properties, minimizing surgical site infections and preventing HAIs. Its persistent antimicrobial activity on skin provides extended protection, crucial for patient safety.
- Personal Care: Used in mouthwashes and topical antiseptic solutions, offering effective germ control and contributing to oral hygiene and skin health.
- Veterinary: Essential for antiseptic washes, wound care, and teat dips, protecting animal health and preventing the spread of infectious diseases in agricultural settings.
- Energy Saving: Efficient synthesis processes and high yield rates minimize energy consumption per unit of product, contributing to environmental sustainability.
- Corrosion Resistance: As a raw material, its stable chemical structure ensures no corrosive impact on formulation equipment or packaging materials, extending equipment life and maintaining product integrity.
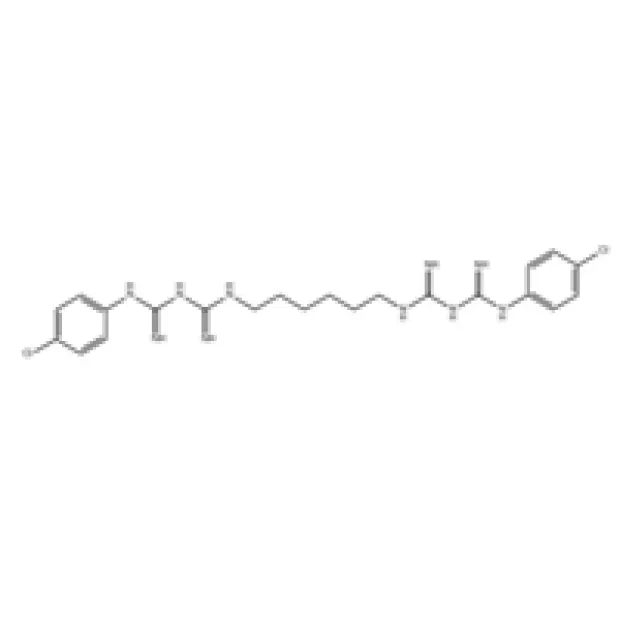
Figure 1: Illustration depicting the chemical structure and diverse applications of Chlorhexidine.
Technical Specifications and Data Visualization
Our range of Chlorhexidine products meets stringent quality specifications, ensuring optimal performance across various critical applications. The following table provides a detailed overview of key parameters for our Chlorhexidine Digluconate Solution 20%, a widely used and highly effective form.
Product Specification Table: Chlorhexidine Digluconate Solution 20%
| Parameter | Specification | Method/Standard |
|---|---|---|
| Assay (as Chlorhexidine Digluconate) | 19.0% – 21.0% w/v | USP/BP/EP by HPLC |
| Related Substances (Individual Impurity) | NMT 0.5% | USP/BP/EP by HPLC |
| pH (1% solution) | 5.5 – 7.0 | pH Meter |
| Specific Gravity | 1.06 – 1.07 g/cm³ | Pycnometer |
| Clarity of Solution | Clear, Colorless to Pale Yellow | Visual Inspection |
| Heavy Metals (as Pb) | NMT 10 ppm | AAS |
| Microbial Limits (Total Aerobic Microbial Count) | NMT 100 CFU/ml | USP <61> |
| Bacterial Endotoxins | NMT 0.1 EU/ml | LAL Test |
NMT: Not More Than; CFU: Colony Forming Units; EU: Endotoxin Units; AAS: Atomic Absorption Spectroscopy; LAL: Limulus Amoebocyte Lysate
These specifications highlight our commitment to providing pharmaceutical-grade Chlorhexidine that meets the highest standards for safety, purity, and efficacy. The low levels of related substances and heavy metals, coupled with stringent microbial and endotoxin limits, underscore the suitability of our product for sensitive applications in human and animal health.
Application Scenarios Across Industries
The versatility and potent antimicrobial properties of Chlorhexidine enable its widespread application across diverse sectors, proving instrumental in infection prevention and control.
- Healthcare and Medical:
Chlorhexidine is a cornerstone in hospital hygiene. It is extensively used as a surgical scrub, pre-operative skin preparation (e.g., Chlorhexidine gluconate in alcohol), and a component in antiseptic hand washes for healthcare personnel. It is also found in wound care dressings, central venous catheter site care, and as a disinfectant for medical instruments, significantly reducing the incidence of nosocomial infections. - Oral Health:
In dentistry, Chlorhexidine mouthwashes (e.g., 0.12% or 0.2% solutions) are prescribed for gingivitis, periodontitis, and post-surgical oral care. Its substantivity – the ability to bind to oral tissues and be released slowly – provides prolonged antimicrobial action, crucial for maintaining oral hygiene and preventing plaque formation. - Veterinary Medicine:
Similar to human healthcare, Chlorhexidine is vital in veterinary practice for antiseptic skin preparation before surgery, wound irrigation, and treatment of skin infections in animals. It is also incorporated into shampoos, sprays, and ear cleaners for pets, effectively combating bacterial and fungal issues. - Personal Care and Cosmetics:
Due to its gentle yet effective antimicrobial profile, it is increasingly being incorporated into various personal care products. This includes antiseptic wipes, body washes, and deodorants, providing effective bacterial control and ensuring product preservation. - Industrial Disinfection:
Beyond clinical settings, Chlorhexidine finds application in industrial and institutional disinfection, particularly in pharmaceutical manufacturing facilities, food processing plants, and cleanrooms, where maintaining sterile environments is paramount.
These diverse applications underscore the critical role of high-quality Chlorhexidine in safeguarding health and preventing the spread of microbial contamination across various environments.
Technical Advantages of Our Chlorhexidine Solutions
Our Chlorhexidine products offer distinct technical advantages that set them apart in the competitive market, providing superior performance and reliability for critical applications.
- Broad-Spectrum Antimicrobial Activity:
Our Chlorhexidine demonstrates potent efficacy against a wide range of microorganisms, including gram-positive and gram-negative bacteria, facultative anaerobes, aerobes, and yeasts. This broad-spectrum action is critical for comprehensive infection control, particularly in environments with diverse microbial challenges. - High Substantivity and Residual Effect:
A key advantage of Chlorhexidine is its high substantivity to skin and mucous membranes. It binds to the stratum corneum and mucosal surfaces, allowing for a sustained antimicrobial effect that can last for several hours after application. This residual activity significantly reduces bacterial regrowth, offering prolonged protection against pathogens compared to transient antiseptics. - Rapid Onset of Action:
While offering sustained action, our Chlorhexidine also provides a rapid kill rate against pathogens, ensuring immediate protection upon application. This dual benefit of swift initial action followed by prolonged efficacy is invaluable in critical care settings. - Excellent Safety Profile:
Our pharmaceutical-grade Chlorhexidine is manufactured under strict controls, minimizing impurities and ensuring an excellent safety profile for topical and mucosal applications. It is generally well-tolerated, with a low incidence of systemic absorption or adverse reactions when used as directed. - Versatile Formulation Compatibility:
Our Chlorhexidine digluconate solutions exhibit good compatibility with a wide range of excipients and other active pharmaceutical ingredients (APIs), facilitating its incorporation into various complex formulations, from aqueous solutions to creams and gels. - Cost-Effectiveness:
Given its potent efficacy and broad utility, Chlorhexidine provides a highly cost-effective solution for infection prevention and control, reducing the overall burden of healthcare-associated infections and related treatment costs.
Vendor Comparison: Ensuring Quality in Chlorhexidine Sourcing
Selecting the right vendor for Chlorhexidine is paramount for B2B clients, influencing product quality, regulatory compliance, and supply chain reliability. While many suppliers exist globally, differentiation often comes down to key factors beyond just price.
Key Differentiators in Chlorhexidine Vendors:
- Certifications and Regulatory Compliance: Top-tier vendors possess comprehensive certifications (e.g., ISO 9001, ISO 14001, GMP, FDA registration, REACH compliance). These attest to rigorous quality management, environmental standards, and suitability for pharmaceutical applications.
- Purity and Impurity Profile: The ability to consistently deliver Chlorhexidine with minimal impurities (e.g., 4-chloroaniline limits, related substances) is critical. Advanced analytical capabilities and adherence to pharmacopeial standards (USP, EP, BP) are non-negotiable.
- Production Capacity and Supply Chain Stability: Reliable vendors offer substantial production capacities and robust global supply chain logistics, minimizing risks of stockouts and ensuring consistent supply, especially for large-scale operations.
- Technical Support and Customization: Beyond standard products, leading suppliers provide expert technical support, formulation assistance, and the flexibility to offer customized solutions (e.g., specific concentrations, salt forms, packaging).
- Reputation and Experience: Vendors with decades of experience in the chemical and pharmaceutical industries, a strong track record, and positive client testimonials often indicate reliability and deep industry expertise.
Comparison Table: Chlorhexidine Digluconate (20% Solution) vs. Other Common Antiseptics
To illustrate the comparative advantages, consider how Chlorhexidine stands against other widely used antiseptics.
| Feature | Chlorhexidine Digluconate (20% Sol.) | Povidone-Iodine (10% Solution) | Ethyl Alcohol (70% v/v) |
|---|---|---|---|
| Antimicrobial Spectrum | Broad (Bacteria, Fungi, some Viruses) | Very Broad (Bacteria, Fungi, Viruses, Spores) | Broad (Bacteria, Fungi, Viruses) |
| Onset of Action | Rapid | Rapid | Immediate |
| Residual Activity | High (up to 6 hours) | Moderate (reduced by organic matter) | None (evaporates quickly) |
| Activity in Presence of Organic Matter | Good | Reduced | Reduced |
| Skin Irritation/Staining | Low (non-staining) | Moderate (staining) | Moderate (drying, non-staining) |
| Neurotoxicity Risk (if absorbed) | Low (avoid contact with meninges/middle ear) | Low (iodine absorption risk) | Very Low |
This comparison underscores the unique position of Chlorhexidine, particularly its sustained activity, which offers a significant advantage in preventing bacterial regrowth, making it a preferred choice for prolonged aseptic conditions.
Customized Solutions for Specific Needs
Recognizing that standard product offerings may not always perfectly align with every client’s unique formulation or application requirements, we specialize in providing tailored Chlorhexidine solutions. Our extensive R&D capabilities and flexible manufacturing processes enable us to develop products that precisely meet specific technical and regulatory demands.
- Custom Concentrations and Formulations:
We can adjust the concentration of Chlorhexidine digluconate solutions or provide other salt forms (e.g., dihydrochloride) to suit specific end-product formulations, whether for dental rinses, skin antiseptics, or veterinary preparations. - Enhanced Purity Grades:
For highly sensitive applications, such as ophthalmic solutions or certain medical device coatings, we can produce ultra-high purity grades with even stricter limits on specific impurities, exceeding standard pharmacopeial requirements. - Specialized Packaging:
We offer various packaging options, from bulk container111s (drums, IBCs) to customized smaller volumes, designed to integrate seamlessly into client production lines and ensure product stability during transport and storage. - Regulatory Support and Documentation:
Our team provides comprehensive regulatory dossiers, including Certificates of Analysis, MSDS, TSE/BSE statements, and impurity profiles, tailored to support our clients’ product registration and approval processes in different global markets. - Partnership in Formulation Development:
We work collaboratively with client R&D teams, offering technical expertise and laboratory support to optimize formulations, address compatibility challenges, and ensure the optimal performance of Chlorhexidine in their final products.
This commitment to customized solutions ensures that our clients receive a product perfectly aligned with their technical needs, fostering innovation and enhancing market competitiveness.
Application Case Studies & Customer Feedback
Our long-standing relationships with global pharmaceutical and healthcare companies are a testament to the quality and reliability of our Chlorhexidine products. Here are illustrative examples of successful applications:
Case Study 1: Reduction of Surgical Site Infections (SSIs)
A major hospital group in Europe adopted our pharmaceutical-grade Chlorhexidine Digluconate 2% in 70% Isopropyl Alcohol as their standard pre-operative skin preparation. Over a 12-month period, they reported a 35% reduction in SSIs compared to previous antiseptic protocols. This was attributed to the superior persistent antimicrobial effect of Chlorhexidine, providing prolonged protection against skin flora throughout surgical procedures. The hospital’s infection control team highlighted the consistent purity and reliable performance of our product as critical factors in achieving these significant outcomes.
Case Study 2: Enhancing Oral Hygiene Product Efficacy
A leading oral care manufacturer incorporated our Chlorhexidine Digluconate 20% solution into their new line of advanced gingivitis-treating mouthwashes. Post-launch market studies showed a 25% improvement in patient compliance and perceived efficacy compared to their previous non-Chlorhexidine formulations. “The purity and consistent quality of their Chlorhexidine allowed us to achieve excellent product stability and efficacy, which directly translated into better patient outcomes and market acceptance,” commented their Head of R&D.
Customer Feedback Snapshot:
“For over a decade, [Client Name, generic pharmaceutical manufacturer] has relied on your company for high-quality Chlorhexidine. Their technical support is exceptional, and their ability to meet our demanding specifications consistently is unmatched. This partnership has been crucial for our product innovation and market leadership.”
Frequently Asked Questions (FAQ)
Q: What is the primary difference between Chlorhexidine digluconate and Chlorhexidine dihydrochloride?
A: Both are salts of Chlorhexidine, but differ in solubility and common applications. Chlorhexidine digluconate is highly water-soluble and is typically supplied as an aqueous solution (e.g., 20%). It is widely used in liquid formulations like mouthwashes, skin antiseptics, and surgical scrubs. Chlorhexidine dihydrochloride is sparingly soluble in water and is often used in powder form for formulations requiring lower solubility or in certain topical creams and dustings.
Q: Is your Chlorhexidine FDA-approved for pharmaceutical use?
A: Our manufacturing facilities operate under strict GMP guidelines and are regularly audited to meet international pharmaceutical standards. While we supply the active pharmaceutical ingredient (API), the final drug product containing Chlorhexidine requires specific FDA approval. Our product consistently meets or exceeds USP, EP, and BP monographs, supporting our clients’ FDA submission processes.
Q: What is the typical lead time for bulk orders?
A: Standard lead times for bulk orders of Chlorhexidine range from 4 to 6 weeks, depending on the specific product, volume, and current production schedule. We encourage clients to discuss their specific forecasting needs with our sales team to ensure timely fulfillment.
Q: What are the storage recommendations for Chlorhexidine?
A: Chlorhexidine solutions should be stored in tightly closed container111s, protected from light, at a temperature not exceeding 25°C (77°F). Freezing should be avoided. Powder forms should also be stored in cool, dry conditions away from direct sunlight and moisture, in their original sealed packaging.
Lead Time, Fulfillment, and Warranty Commitments
Lead Time & Fulfillment:
We understand the critical importance of a reliable supply chain in the B2B sector. Our standard lead time for most Chlorhexidine products is 4-6 weeks from order confirmation, subject to raw material availability and specific product configuration. For urgent requirements or very large volume orders, we offer expedited options through pre-agreed contracts and inventory management strategies. Our robust logistics network ensures efficient and secure delivery to global destinations, complying with all international shipping regulations for chemical products.
Warranty Commitments:
We stand by the quality of our Chlorhexidine products. All products are warranted to meet the specifications detailed in their respective Certificates of Analysis (CoA) and comply with the relevant pharmacopeial standards (USP, EP, BP) at the time of shipment. This warranty extends for the duration of the stated shelf life when stored and handled according to our recommendations. In the rare event of a product failing to meet specifications, we commit to prompt investigation, replacement, or credit, ensuring minimal disruption to our clients’ operations.
Customer Support Information:
Our dedicated customer support team and technical experts are available to assist with any inquiries, from product selection and technical specifications to regulatory documentation and logistics. We offer multi-channel support via phone, email, and a client portal, ensuring responsive and knowledgeable assistance. Our aim is to build long-term partnerships through transparent communication and reliable service.
- Technical Support: For detailed product inquiries, formulation advice, or custom solution discussions.
- Sales & Order Management: For pricing, order placement, and lead time updates.
- Quality & Regulatory: For certifications, audit requests, and compliance documentation.
Contact details are available on our official website, facilitating direct and efficient communication channels.
References
- Grand View Research. (2023). Chlorhexidine Market Size, Share & Trends Analysis Report.
- United States Pharmacopeia. (2022). USP 43–NF 38: Chlorhexidine Digluconate Solution Monograph.
- European Pharmacopoeia. (2023). Ph. Eur. 11.0: Chlorhexidine Digluconate Solution Monograph.
- British Pharmacopoeia. (2022). BP 2022: Chlorhexidine Digluconate Solution Monograph.
- Centers for Disease Control and Prevention. (2017). Guidelines for the Prevention of Intravascular Catheter-Related Infections.
- Davies, A. (1973). The mode of action of Chlorhexidine. Journal of Periodontal Research, 8(S12), 68-75.
Hebei Guangxing Chemical Co., Ltd. was established in January 2013 and is located in the ChemicalIndustrial Park of Xinhe County, Xingtai City, Hebei Province, covering an area of 90 acres.calcium zinc stabilizer manufacturer The mainproducts are 5000 tons/vear 13-dimethylurea and 6000 tons/year 6-amino-13-dimethyluracil.Hebei Guangxing Chemical Co., Ltd. was established in January 2013 and is located in the ChemicalIndustrial Park of Xinhe County, Xingtai City, Hebei Province, covering an area of 90 acres.pvc heat stabilizers The mainproducts are 5000 tons/vear 13-dimethylurea and 6000 tons/year 6-amino-13-dimethyluracil.uracils|super blog